| Figure 1. Exercise! |
 |
| Image obtained from http://macnavs.ca/ultimate_frisbee.jpg |
Exercise has benefits, not only for cardiovascular and general physical health, but it also plays a role in brain health.[1] On top of regular aging, which generally brings a cascade of health problems - including decline of cognitive function, but there are also multiple brain disorders which impair cognition. [2] Long term training and regular aerobic exercise, result in improvements of the cognitive function of healthy individuals, as well as that of older people whose cognitive function is declining or impaired.[3] The mechanisms through which exercise affects cognition are not yet well understood, and are currently still being investigated. Improvements can be observed in various aspects of cognition, therefore, physiological changes must be taking place at the different brain substrates responsible for those functions. Thus, emphasis has been placed in the role that neurotrophins may play in bringing about these effects, since they have brain wide interactions, play a role in dendritic growth and long term potentiation, and their levels fluctuate in response to exercise. [2]
Video neatly outlining the extensive benefits of exercise for health in general.
|
Table of Contents
|
1. Cognitive Benefits
Aerobic exercise brings a variety of cognitive improvements, spanning working memory, executive control, and attention. Although there seem to be some mixed results regarding which areas of cognition benefit from exercise, those variations are likely due to differences in testing procedures. The effects of exercise on cognition are measured by evaluating performance in tasks that target different aspects of cognition, and, in general, comparing performance pre and post aerobic exercise training, or high-fit versus low-fit participants. Several studies, concurrently, look at Event Related Potentials (ERP’s), which measure neuroelectrical patterns that occur in preparation for, and response to, an event. [4] Different parts of the ERP curve indicate different characteristics of the response, such as amount of attentional resources allocated, and efficiency and speed of stimulus identification and classification. [5] ERP’s have very high temporal resolution, and can show brain activity changes related to exercise, even if behaviour seems unaffected. [6]
1.1 Associated with Prefrontal and Cingulate Cortices
Working memory and executive control are mainly functions associated with prefrontal cortex and anterior cingulate gyrus.[8][13] Executive function is still a mildly broad term, referring to an individual’s ability to devise, follow and modify strategies to achieve a goal. [9] Many studies have looked at how executive function and working memory are affected by exercise, and while findings vary slightly from one paper to another, those differences are might be mainly due to age discrepancies. When segregating their findings into broad age groups, a more coherent picture of the relationship between exercise and cognition becomes evident.
Young Children
Tests performed on younger children displayed improved reaction time and accuracy in response inhibition tasks (such as Eriksen Flanker task), which recruit executive control functions. Furthermore, conditions which require greater executive control engagement displayed greater improvements following exercise. ERP measurements also suggest that exercise improves these prefrontal cortex-dependent tasks by facilitating better allocation of attentional resources in preparation for the task. [5] While there is a small exercise-dose effect on the magnitude of cognitive changes observed, this is usually not significantly different. These benefits of exercise have been show to translate into better academic performance, and increased prefrontal cortex activity (observed through fMRI). [10]
Preadolescents and Adolescents
When comparing high-fit with low-fit individuals in this age group, the same trends concerning inhibitory control are observed as in younger children. In addition, response accuracy is maintained across trials and difficulty in high-fit compared to low-fit participants. Furthermore, ERP analysis measured over the anterior cingulate cortex depicts a smaller threshold in detection and correction of erroneous behavior. [4] Thus, fitness makes response monitoring (inhibition) more effortless, thereby increasing efficiency of executive control. [6] Overall, higher-fit individuals in this age group display better flexible modulation of cognitive control, faster stimulus processing – associated with working memory updating, – and decreased response conflict; all of which indicate more flexible modulation of executive function. [6] [7]
Young Adults
when comparing performance of fit versus sedentary individuals, on tests that engage working memory maintenance and place great demands on executive control, fit participants display improved reaction times, attentional allocation, and working memory updating. [1] [12] In addition, ERPs show that, especially when task conditions are more demanding, fit individual have lower attentional costs associated with task switching, and more efficient executive function overall. [1] Tests with the Tower of London task, which involve planning and problem solving, and thereby greatly engage the prefrontal cortex, also show improvements associated with exercise. [9]
| Figure 2. |
 |
| Adapted from Fig. 1, from k. Erikson et al (2011). Changes in hippocampal volume as a function of exercise. Thalamus and Caudate nucleus volumes included as controls |
Older Adults
Older adults show age-related decrease in anterior prefrontal cortex sustained activity in task switching tasks, compared to healthy young adults. [14] After extended exercise training, performance on those same tasks can be improved to a great extent. [11] Tests following short term aerobic exercise also exhibit improved performance and reaction times in subsequent short term memory tasks, coupled with enhanced activity in the prefrontal cortex. [13]
Another study found a positive correlation between increases in physical activity and enhanced episodic memory recall over time. While episodic memory is usually associated with hippocampal function, in this particular study, participants exhibited increased gray matter volume in the prefrontal and cingulate cortices. [15]
1.2 Associated with Hippocampus
Spatial memory is highly associated with hippocampal function, and positively correlates with anterior hippocampal volume. [16]
In older adults, higher baseline fitness correlates positively with faster reaction times and increased accuracy on spatial memory tasks. Following long term exercise conditions, older individuals who take part in aerobic exercise display increased hippocampal volume and memory performance, compared to baseline, and also when compared with age matched individuals who participate in non-aerobic exercise.[16]
Studies performed with adolescents also show enhanced performance in relational memory between item and background, for fit adolescents versus sedentary age mates.[17]
It is important to note that, especially at younger ages, an individual’s environment plays a critical role in shaping brain structure and connectivity, since these are still under development and undergo great changes before adulthood. Taking this, and the effects of exercise on cognition into consideration, individuals would benefit from starting a regular physical activity schedule from and young age, and maintaining high fitness levels throughout their lifetime.
2. BDNF
Brain-derived neurotrophic factor (BDNF) belongs to a family of proteins, coined neurotrophins, which are considered crucial for the development, differentiation and maintenance of neurons in the CNS and PNS. [2] All the proteins in this family display similar molecular structures, differing only in a few domains associated with binding of different receptors. [2] BDNF structure and pathways are extremely similar in humans and mice[2], such that experiments performed in mice can be directly translate to humans, and effectively used to elucidate underlying mechanism associated with it.
BDNF is not only produced by neurons, but also by astrocytes, Schwan cells and fibroblasts, and is therefore widely spread throughout the nervous system. [2] Functionally, BDNF is generally associated with neural and synaptic plasticity, neurogenesis and LTP, and protection against neuronal degradation. [18][19]
2.1 Mature vs. Immature BDNF Function
When BDNF gene expression is triggered, the molecule that is initially synthesized is an immature precursor of BDNF, referred to as proBDNF, and it requires another transformation to achieve the fully matured from, denoted mBDNF. These two BDNF variants have different, and even opposing, functions in CNS. In fact, while mBDNF has been shown to facilitate synaptic transmission, dendritic and axonal growth, and cell survival; proBDNF is highly associated with synaptic withdrawal and neuronal cell death. [2]These two BDNF variants might then be thought of complementary, and because of the extreme consequences of each, their relative concentrations must be tightly regulated.
2.2 Pathway
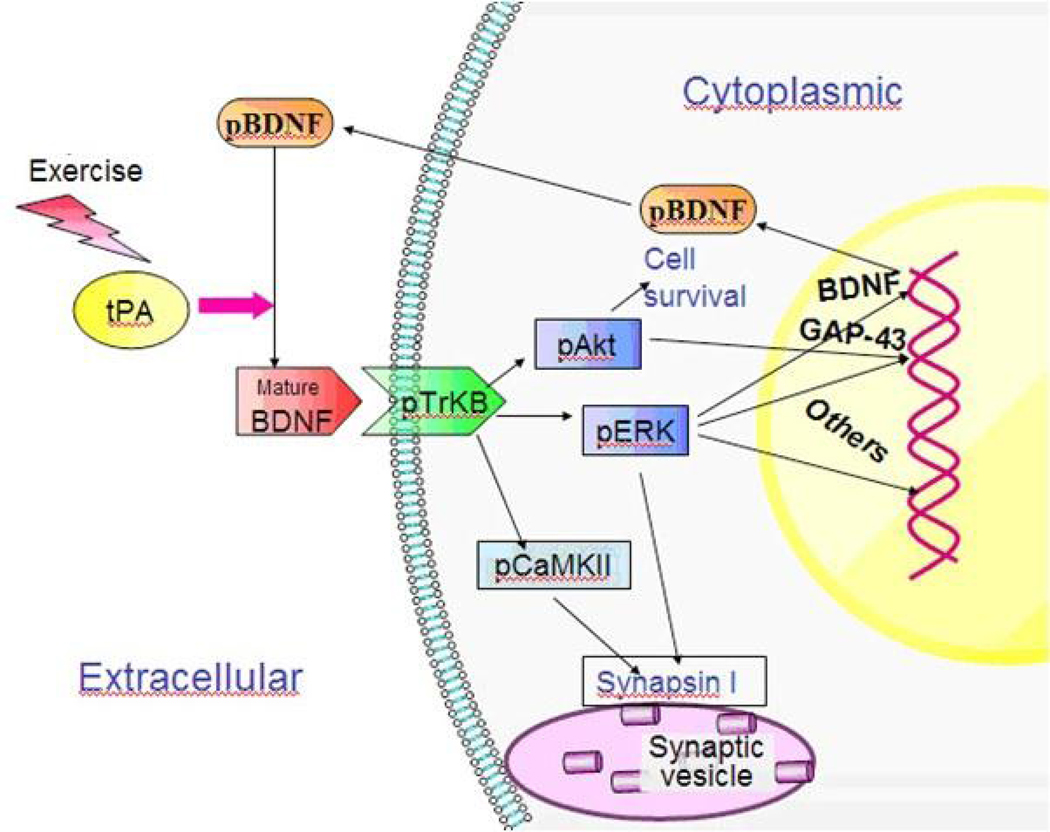
Following the synthesis of proBDNF, a tissue-type plasminogen activator (tPA) is necessary to form mBDNF, by activating a plasminogen which then cleaves the precursor molecule. [18] If not cleaved, pro BDNF will go on to bind p75NTR receptors, which takes part in a signaling cascade associated with cell death or synaptic withdrawal. In contrast, mBDNF goes on to bind TrkB receptors [18], resulting in downstream signaling which facilitates cell survival and growth previously mentioned. These downstream signals ultimately affect SynapsinI synthesis and phosphorylation via pCamKII and pERK signaling, and growth-associated protein 43 (GAP-43) expression (see Figure 3) – all of which are important markers of synaptic plasticity. [18]
SynapsinI is a protein expressed in axon terminals, and I facilitates neurotransmitter release, axonal growth, and synaptic connection maintenance. [18] It’s activation is mediated by CaMKII and pERK signaling, which in turn are affected by mBDNF-bound TrKB. pAkT is another protein affected by mBDNF bound to its receptor, and it is associated with the more complex mechanisms of cell survival.[18]
2.3 Aging, Exercise and BDNF
Multiple studies, with both humans and rodents, have shown that levels of circulating BDNF decrease with typical aging, as well as in individuals afflicted with dementia-related disorders. [2] [3] This decrease in BDNF is highly correlated with the increased deficits that accompany aging, cognitive decline and loss of structural integrity. [3][18][20]
Rodents and humans alike exhibit increased BDNF levels following exercise bouts [12][22], and higher levels of BDNF correlate with better cognitive performance.[9][20][21] [30]
A particular study, performed by Q. Ding and colleagues, shows, not a correlation, but the direct effect of BDNF on exercise-induced hippocampal cell proliferation, associated cognitive function. First, they show that BDNF is increased by exercise, in rodents, and that the end result is increased cell proliferation in the dentate gyrus of the hippocampus. Then, formation of mBDNF is prevented by using a drug that blocks tPA activity, and the animal is allowed to wheel-run. Results show that by preventing BDNF maturation, hippocampal cell proliferation is abolished.[18] Since pro BDNF levels increased, this indicates that exercise stimulates BDNF synthesis, and that tPA, along with mBDNF, plays a crucial role in mediating the effects of exercise on hippocampal cell proliferation, and therefore its effects on hippocampus-based cognitive function.
3. Dopamine
The idea that dopamine may play a role in cognitive functions associated with prefrontal cortex activity may have stemmed from observing the effects of Parkinson's Disease (PD) on working memory.[31] PD patients present with both motor and cognitive deficits, and the hallmark of the disorder is a rapid degradation of dopaminergic neurons. In fact, increasing dopamine levels improves cognitive performance, however, once threshold levels are reached the effect is reversed.[25]
3.1 COMT Gene
Catechol-O-methyltransferase (COMT) is an enzyme involved in eliminating dopamine from the synaptic cleft.[29] A Val158Met polymorphism on the gene leads to synthesis of an unstable protein, which results in reduced enzymatic activity[26], thus dopamine remains in the synapse longer. Heterozygotes have intermediate phenotype for this gene. [29]
COMT regulates dopamine levels mainly in the prefrontal cortex, but also in some subcortical areas.[27]
3.2 Modulation of Cognitive Benefits
Using the COMT polymorphism as an indicator of dopamine availability in the CNS, the relationship between dopamine, exercise and cognition can be better elucidated. Met carriers show a trend of outperforming Val carriers in response inhibition task, but don't reach statistical significance. [29]
If dopamine availability modulates the effects of exercise on cognition, it must play a more indirect role than simply saying that increased dopamine levels result in greater cognitive changes following exercise.
4. Neurometabolite Changes
Neurometabolites are molecules associated with energy metabolism in the nervous system, and some of them also play pivotal roles in neuronal cell structure and viability.[((bibcite r33)]
The concentrations of these molecules can be measured via Magnetic Resonance Spectroscopy (MRS), since each neurometabolite has a characteristic peak.
4.1 N-Acetylaspartate
N-Acetyl-Aspartate (NAA) is an amino acid and a neurometabolite particular to the nervous system, commonly found in neuronal soma.[23] This amino acid is synthesized in the mitochondria, Where it is related to ATP production.[24] NAA levels in the CNS are, therefore, indicative of neuronal intergrity and metabolic efficiency.[24]
Both typical aging and cognitive disorders are associated with a decrease in NAA concentrations in the brain, likely due to loss of neuronal density.[23]
BDNF studies have provided a strong link between exercise and cognitive benefits associated with the hippocampus. However, many of the cognitive benefits associated with exercise and fitness are based on prefrontal cortex activity. NAA is a strong candidate to elucidate the underlying mechanism of these effects, as aerobic exercise is positively correlated with an increase in NAA concentration in frontal gray matter, including the anterior cigulate cortex.[23]In fact, fitness measures can accurately predict NAA concentrations in these brain areas.[23] In addition, regular aerobic exercise has the potential to offset age related decline in NAA concentration, and the cognitive decline associated with it.[31] Because NAA is predominately found in neuronal cell bodies, it can be seen as an indicator of neuronal density,[31] and it's increase can be linked to the increase in volume associated with the particular brain areas associated with cognition.[22]
4.2 Choline
Choline is needed for acetycholine synthesis and cell membrane formation.[24]It's concentration in the occipital gray matter, encompassing the posterior cingulate gyrus, increases in response to short- and long-term exercise, which leads to speculation that fitness helps maintain cell structure in these areas.[23]
It is important to note that the studies discussed in this neurometabolite section targeted and measured specific areas of the brain, based on the fact that those areas have been shown to be implicated in cognition in previous literature. This adds the implication that neurometabolite concentrations in other brain areas and their possible connection to exercise have not been explored.
See Also
Cognitive Benefits of Videogames
The Effects of Exercise on Stress
Copy Number Variations - Proteins Affecting BDNF Secretion
miRNA in Alzheimer's Disease
Music & Alzheimer's Disease - Neuroprotection
Memory Failure
Effects of Lifestyle on Prevalence of Alzheimer's Disease
Plasticity in the Athletic Brain

Hey I see that this page is still under construction, however the 2nd image is not visible
Everything looks great so far, keep it up! Hopefully, the final product will motivate me to hit the gym more often.
It's amazing how people these days don't get out more, although I doubt many people would be doing the crocodile frisbee catching sport. Hopefully this post will get us out of our chairs more and into the gym more! Just to let you know, you have an extra period after your last citation in the introduction. Keep up the great work!
Hey i like your first crocodile figure up there, it's SO catchy!! Can I make a link to your page for the part that u talked about ERP's?
Thanks, I honestly put it up just for testing the code and all.. but maybe i should leave it? :)
Feel free to link it.
Oh no, your crocodile figure is not visible! Question: Is there a certain amount of exercise one needs to engage in to see changes in cognition? I.e., is there a threshold of some sort?
Findings related to that vary a bit . Some studies find that cognition is improved after a single bout of aerobic exercise (some as short as 30 mins), along with increases in central and peripheral BDNF, although, to my understanding this is a short term effect. there is a particular study which specifically states that it is long term fitness, and not just exercising now and then, that improves cognition. from these findings, I would say that if you exercise at least a few times a week, for about 1hr, your cognitive performance should improve. Hope that helps. I'll be uploading more sections soon.
The intro was so much more enjoyable to read with that crocodile image in the periphery. Thought this video might be relevant: http://www.youtube.com/watch?v=aUaInS6HIGo
It looks good so far. What is the link between BDNF and neurogensis? To what extent can one promote neurogensis though increased levels of BDNF(via exercise)? How does neurogensis improve cognition.
Also, you can link with my Neurowiki as I am looking into exercise and the role of BDNF, neurogenesis in reducing stress levels! It's an incredibly interesting topic!
Looks pretty good right now, I think the first picture is great, but the next two have a formatting issue I think, they may need to be moved further apart. Very interesting to read so far!
I agree, after sitting down and reading through this page, it makes me want to get up and active! That in itself is a good thing :)
When talking about PD and COMT, link it to one of the neurowikis on PD.
Thank you for linking to my page! (Cognitive Benefits of Video Games) I think I remember your presentation a few months back and thinking how our topics can definitely have some sort of connection. Your page is wonderfully done with relevant images. I don't have much to comment on the content other than it's very well written. Format wise, under section 2.3, the sources are not numerical (18,19,2) small thing lol. And lastly references maybe bold and italicize to fit Nature formatting? All small nit picky things, good job on your page~